SPONSOR PORTAL
Need Assistance?
EMR Access
Request Study Folder
Change Organization
Link Study Folder
Monitoring Visit
Feasibility

ABOUT CIC
- A “one-stop” centre to facilitate the conduct of safe and credible clinical research in drug, medical devices and cell & gene therapy
- A point of contact to access an integrated network of experienced clinical investigators
- Providing global standards of Good Clinical Practice (GCP), training and Quality Assurance, using a global Standard Operating Procedure
- Accessibility to a large pool of research subjects
- Sophisticated and modern infrastructure for multicentric clinical trials
CORE SERVICES
- Feasibility surveys for new clinical trials
- Budget planning for clinical trials
- Clinical Trial Agreement vetting
- Clinical trial fund management
- Calibrated equipment & well maintained facility for execution of world standard clinical research.
- Conduct GCP and other relevant clinical trial training courses.
- Facilitate better clinical trial outcome by guiding new investors in clinical trial processes, connecting them to collaborators and coordinating activities related to the clinical trial

100
Investigators
50
Awards
50
Study Coordinators
300
Active Trials
20
Administrative Staffs
20
Therapeutics
15
MoU Collaboration
300
Number of Sponsors
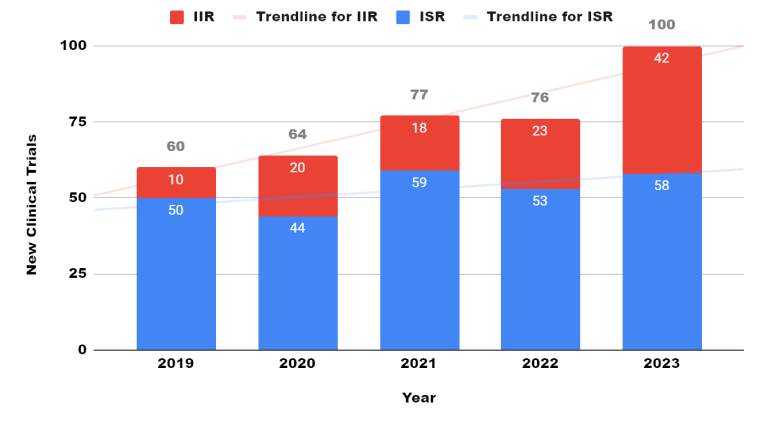
New Clinical Trials: Industry Sponsored Research (ISR) and Investigator Initiated Research (IIR) in the year 2019 - 2023
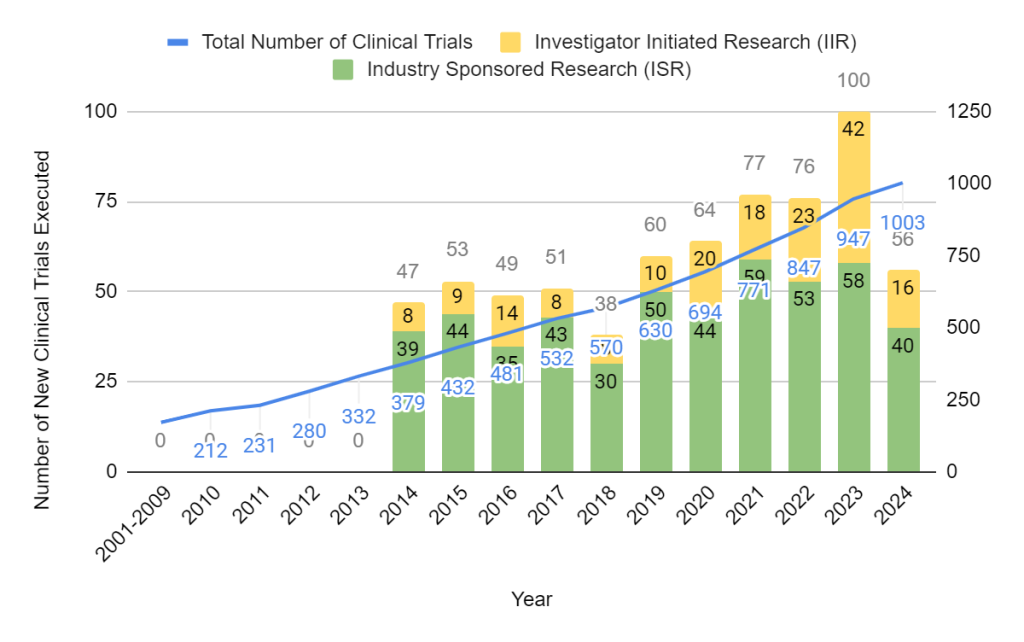
CIC Clinical Research Over the Years
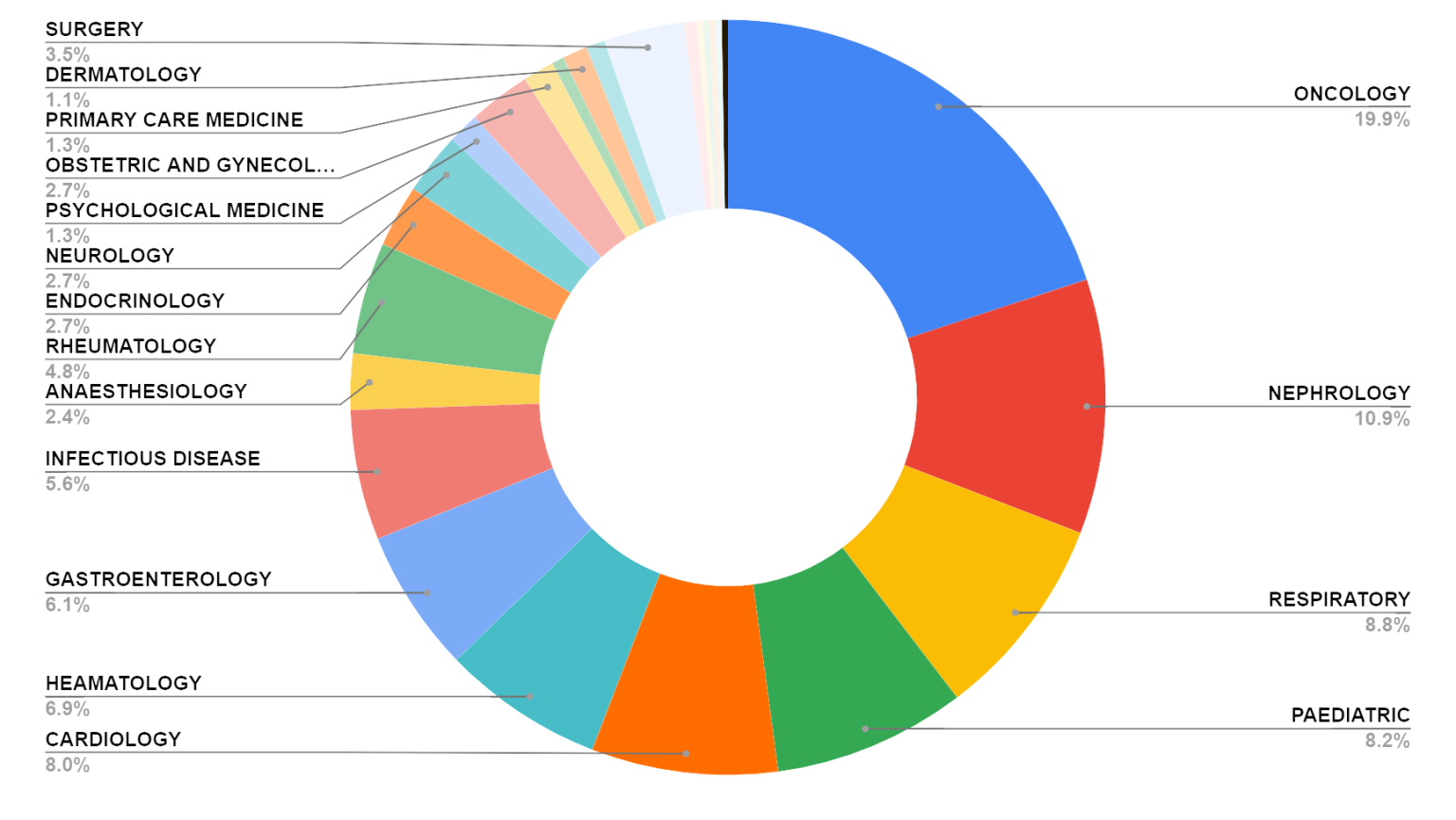
Number of New Industry Sponsored Research Conducted Based on Therapeutic Area Year 2023

The Percentage of Active Study by Therapeutic Area